Greater availability of essential medicines in the EU
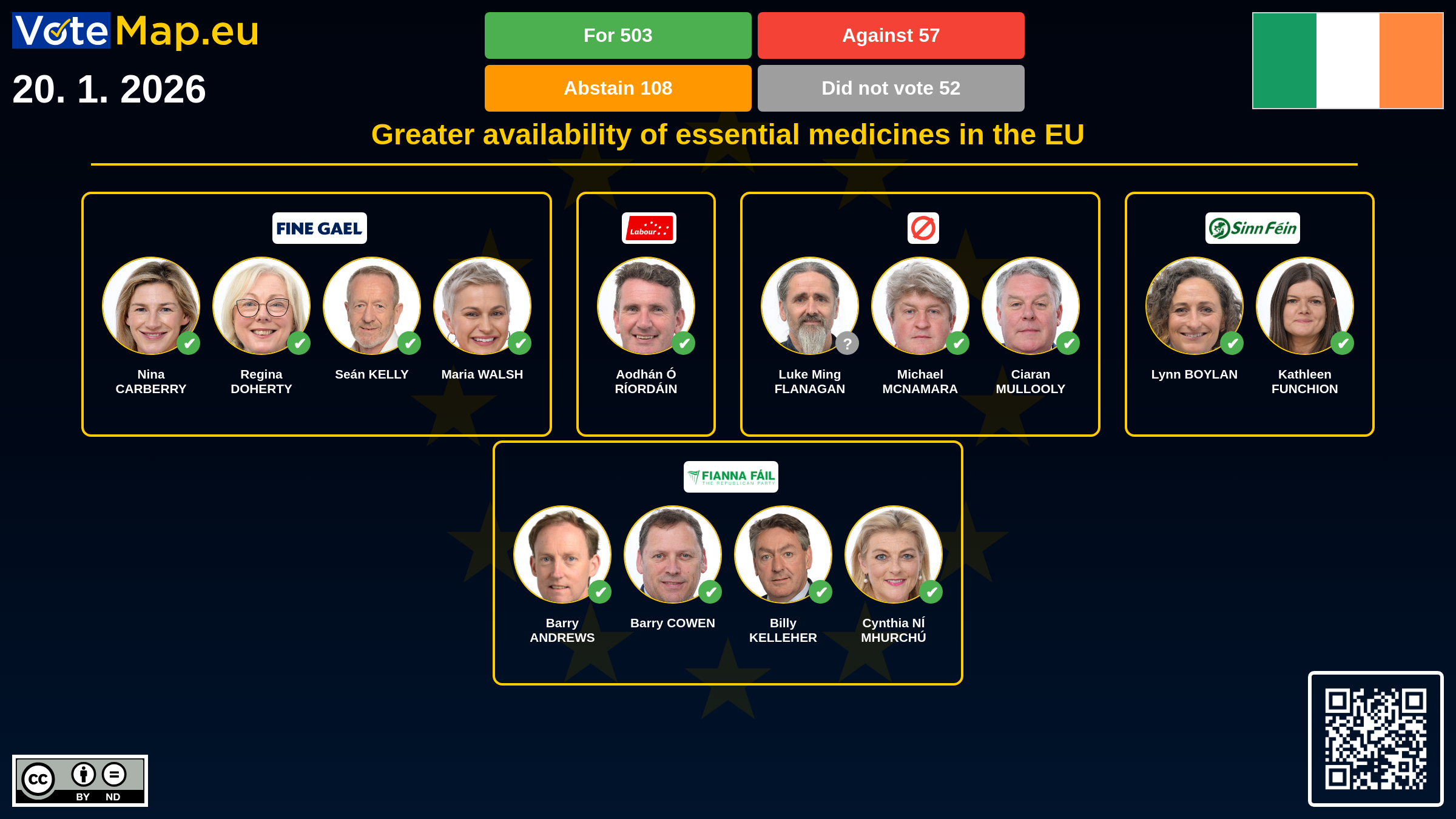
MEPs have approved proposals aimed at increasing the availability of so-called critical medicines and strengthening the competitiveness of the European pharmaceutical industry. According to Parliament, more than half of medicine shortages in the EU today are due to production problems, often outside the Union. Investment in production capacity in the EU is to be a key pillar of the strategy. Parliament supports the creation of strategic manufacturing projects in Europe, which should receive priority public support. Companies that draw on these funds would also have to guarantee supplies primarily for the European market. The changes are also to be reflected in public procurement. When purchasing medicines, the decision should no longer be based solely on the lowest price, but also on the security of supply and the place of manufacture. The aim is to favour manufacturers who produce a significant proportion of their medicines directly in the EU – a clear 'Buy European' approach. At the same time, Parliament supports joint cross-border purchases of medicines between Member States, particularly for rare, expensive or specialised products. The proposals also include better coordination of national medicine stocks, including the possibility of redistributing them in the event of an acute shortage. The European Parliament's position will now serve as a basis for negotiations with Member States on the final form of the rules to ensure that essential medicines are available in the EU in a timely manner and in sufficient quantities.
We collect and visually present data publicly available at the page of European Parliament. Votemap.eu denies any responsibility for possible inconsistencies of the data or its changes after the publication.
Descriptions are created using DeepL Translate machine translation. We apologize for any possible imperfections or inconvenience.





























